and there are a lot of them taking on various vital funcions within a
living organism.
But as I work my way to putting together a reasonable introduction to
said proteins, I am quickly drawn to clarifying in my own mind various
concepts in biochemistry that come from chemistry, and even physics.
So let's deal with that first.
Ph value is a negative logarithm.
source: Math is Fun
So an acid has a larger number of protons to give than a base, which results
in a lower Ph value because that value is how many times 1 should be divided by
10.
And what gives with hydrogen, which is electrically neutral with one proton (positive
charge) and one electron (negative charge), but hooks up with oxygen to form water as H2O.
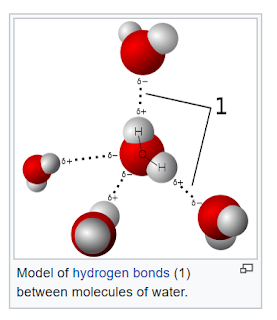
In a compound, the first electron shell actually accepts two electrons before going to
a different shell further out. Water itself is stable because of secondary attractions
between water molecules. To be precise, it is slightly acidic.
source: wikipedia
Actually, hydrogen is quite a heavy player is cosmology, responsible for the formatiion
of other elements.
Elements sometimes have neutrons in their nuclei; this is for stability. In physics, a neutron
is seen to disintegrate as a proton, an electron and an antineutrino, with a mean lifespan of 15
minutes.
Proteins are polypeptides - chains of amino acids - held together by
peptide bonds. Indeed, if you have a chain with more than 100 elements, it
is no longer a polypeptide but a protein. But proteins are also more built up,
with secondry, tertiary and even a fourth level of structure to assume their roles.
source: Wikipedia
The name protein means that it is essential. The original Dutch name given was
'wortstof', a combination of root and stick. Quite telling really, because proteins are
built up from coplanar units that hook up. And produce water as a by-product.
Proteins are built up from amino acids (an amine contains nitrogen).
Our players on the periodic table:
source: Wikipedia
https://www.wikihow.com/Study-the-Basics-of-Biochemistry




No comments:
Post a Comment